Biologics in Severe Asthma: How Anti-IgE and Anti-IL-5 Therapies Work
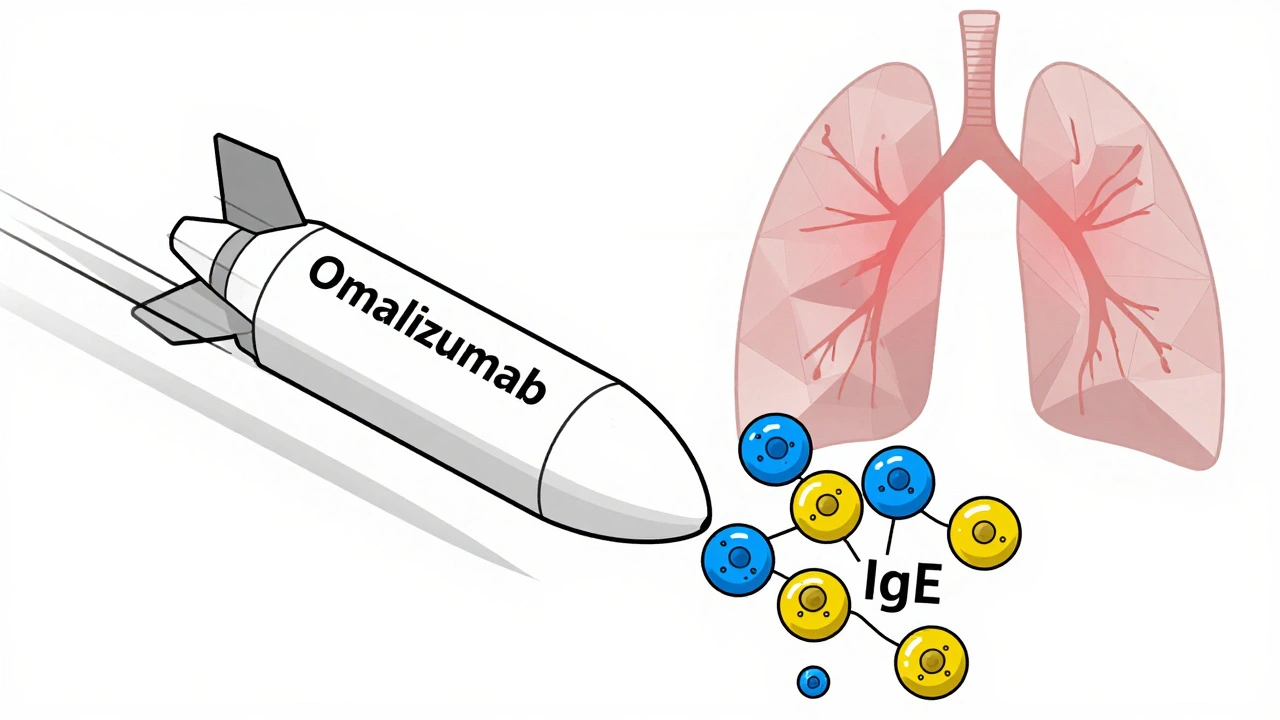
What Are Biologics for Severe Asthma?
Severe asthma doesn’t respond well to standard inhalers, even when used correctly. For these patients, biologics offer a new path forward. These aren’t regular pills or sprays. They’re lab-made proteins-monoclonal antibodies-that zero in on specific parts of the immune system driving inflammation in the lungs. Think of them like precision missiles instead of scatter shots.
Two major classes of biologics dominate treatment today: anti-IgE and anti-IL-5. Omalizumab (Xolair) targets immunoglobulin E, the antibody responsible for allergic reactions. Anti-IL-5 drugs-mepolizumab (Nucala), reslizumab (Cinqair), and benralizumab (Fasenra)-block interleukin-5, a signal that tells eosinophils (a type of white blood cell) to swarm the airways and cause swelling.
These treatments are only for people whose asthma stays out of control despite high-dose inhaled steroids and long-acting bronchodilators. They’re not first-line. They’re for when everything else has failed.
How Anti-IgE Therapy (Omalizumab) Works
Omalizumab is the original biologic for asthma, approved back in 2003. It binds to free IgE in the bloodstream before it can attach to mast cells and basophils. When IgE can’t latch on, those cells don’t release histamine and other inflammatory chemicals that trigger wheezing, coughing, and tightening of the airways.
This therapy works best for people with allergic asthma. You need proof of allergy-either a positive skin test or elevated specific IgE levels to things like dust mites, pet dander, or pollen. Your total serum IgE must be between 30 and 1500 IU/mL. If you don’t have allergies, omalizumab won’t help.
It’s given as a subcutaneous injection every two to four weeks, depending on your weight and IgE level. Most people start feeling better after 12 to 16 weeks. Some notice improvements sooner, but patience is key. You can’t skip doses. The effect builds over time.
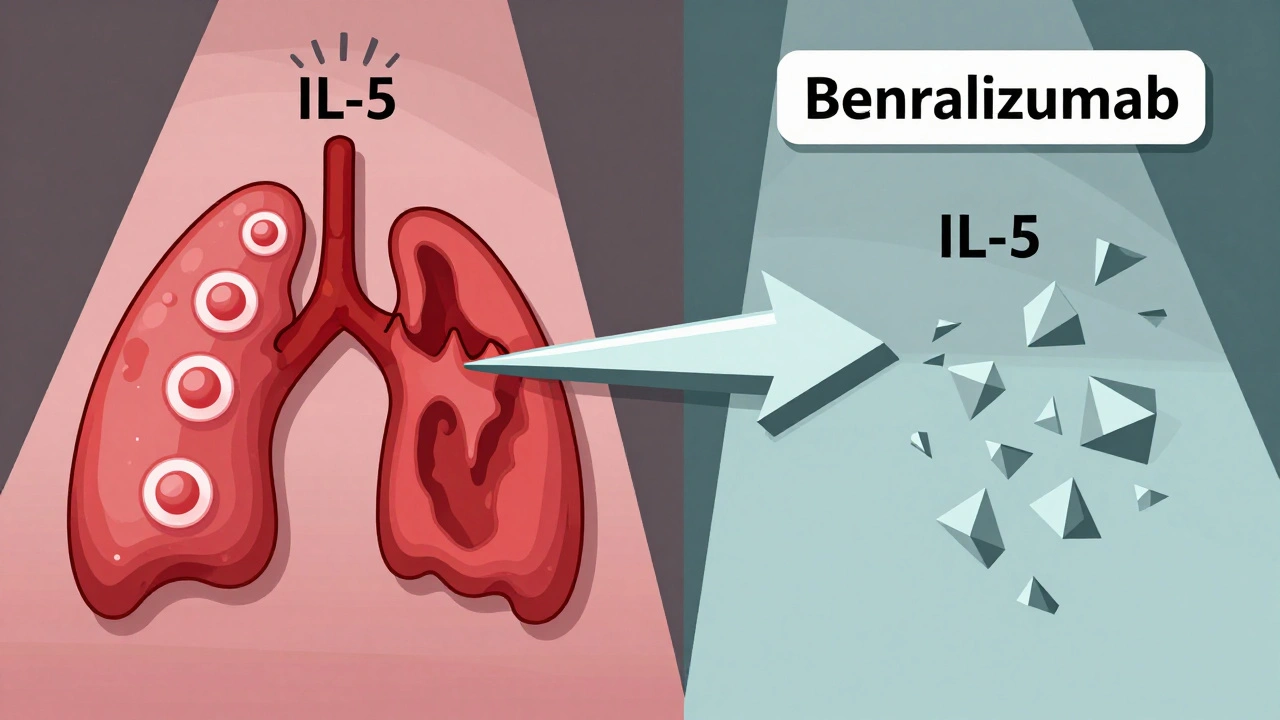
How Anti-IL-5 Therapies Target Eosinophilic Asthma
Not all severe asthma is allergic. Some people have high levels of eosinophils in their blood or lungs-often without clear triggers. This is called eosinophilic asthma. That’s where anti-IL-5 drugs come in.
Mepolizumab and reslizumab directly bind to IL-5, stopping it from activating eosinophils. Benralizumab goes a step further: it attaches to the IL-5 receptor on eosinophils themselves and triggers their destruction. This means eosinophil counts can drop by over 95% within 24 hours after a benralizumab shot.
To qualify, you typically need a blood eosinophil count of at least 150 cells/μL in the past year-or 300 cells/μL if you’ve had recent flare-ups. These drugs don’t care if you’re allergic. They care about your eosinophils.
Dosing varies: mepolizumab and benralizumab are injected under the skin every 4 weeks (benralizumab switches to every 8 weeks after the first three doses). Reslizumab requires an IV infusion every 4 weeks, which means a clinic visit each time.
Real-World Results: What Patients Actually Experience
Clinical trials show biologics reduce asthma attacks by 40% to 60%. That’s not just numbers-it means fewer ER trips, fewer hospital stays, and less need for oral steroids like prednisone.
One patient in the UK, on mepolizumab, went from four emergency visits a year to none. Another, on benralizumab, stopped taking daily steroids and started hiking again. But it’s not universal. About 30% to 40% of people don’t respond at all. That’s why matching the right drug to the right patient matters.
Side effects are usually mild: soreness at the injection site, headache, or a sore throat. About 1 in 10 people get these. Anaphylaxis is rare-around 1 in 1,000 doses-but more likely if you’ve had severe allergies before. That’s why the first few doses are given in a medical setting.
Some people report joint pain or fatigue. One Reddit user stopped benralizumab after three shots because of severe joint pain, even though their asthma improved. Others swear by it. Individual responses vary.
Who Should Get These Treatments-and Who Shouldn’t
Before starting any biologic, your doctor needs to confirm three things:
- You’re using your inhalers correctly and consistently.
- Your asthma is truly severe-frequent attacks, hospitalizations, or steroid dependence.
- You have the right biomarker: IgE for omalizumab, eosinophils for anti-IL-5 drugs.
If you’re a non-allergic, non-eosinophilic asthmatic, these drugs won’t help. Trying them anyway is a waste of time and money. Biomarker testing isn’t optional-it’s required.
Also, biologics don’t replace your regular asthma meds. You still need your inhalers. They’re add-ons, not replacements.
Children as young as six can get omalizumab. Anti-IL-5 drugs are approved for ages 12 and up. No one under 6 gets biologics yet, though trials are ongoing.
Cost, Access, and Insurance Hurdles
These drugs are expensive. Annual costs range from $25,000 to $40,000 in the U.S. In the UK, they’re available through the NHS, but access isn’t automatic. You need to go through a specialist asthma clinic and prove you meet strict criteria.
Insurance approval can take 14 to 21 days. Many patients wait months before getting started. Manufacturer support programs help with co-pays and provide nurses to train you on self-injection.
Most people learn to inject themselves after two or three supervised sessions. Auto-injector pens make it easier than you’d think. But you still need to keep up with appointments and blood tests to monitor eosinophils or IgE levels.
The Bigger Picture: Where This Is All Heading
Biologics have changed the game for severe asthma. But we’re still early in this journey. Tezepelumab (Tezspire), approved in 2021, targets TSLP-an upstream protein that triggers multiple inflammatory pathways. It works even if you don’t have high eosinophils or IgE. That’s a big deal.
Now, researchers are testing twice-yearly injections. Imagine getting a shot only twice a year instead of every month. Phase 3 trials are underway. Oral biologics are also in development, though none are approved yet.
The goal isn’t just to reduce attacks. It’s to restore quality of life. People on these drugs report sleeping better, working full-time, and not fearing the next cough. That’s the real win.
But cost and access remain huge barriers. Only 1-2% of eligible patients in the UK and Europe are on biologics. The gap between who could benefit and who actually gets treated is wide.
What Comes Next?
If you or someone you know has severe asthma that won’t quit, ask your specialist about biomarker testing. Don’t assume you’re not a candidate. Many people are turned away because they didn’t get tested properly.
Keep track of your attacks, your steroid use, and your lung function. Bring that data to your appointment. It helps your doctor decide if a biologic makes sense-and which one.
And remember: biologics aren’t magic. They’re powerful tools, but they work best when paired with good asthma management, avoidance of triggers, and regular follow-ups.
Can biologics cure severe asthma?
No, biologics don’t cure asthma. They control it by targeting specific parts of the immune system that cause inflammation. Most patients still need to use inhalers and avoid triggers. But many achieve near-normal lung function and drastically reduce flare-ups.
How long does it take for anti-IgE or anti-IL-5 biologics to work?
Improvement usually starts between 4 and 16 weeks. Some notice fewer symptoms within a month, but full benefits often take 3 to 6 months. Don’t stop treatment if you don’t feel better right away-this isn’t an instant fix.
Are there any long-term risks with using biologics for years?
So far, biologics have been used safely for over 20 years in some patients. The most common long-term concern is potential increased risk of parasitic infections, but this is rare in developed countries. There’s no clear link to cancer or organ damage. Ongoing studies are tracking patients beyond 5 years.
Can I switch from one biologic to another if the first one doesn’t work?
Yes, switching is common. If omalizumab doesn’t help, your doctor might try mepolizumab or benralizumab, especially if your eosinophil count is high. The reverse is also true-if an anti-IL-5 drug fails and you have high IgE, omalizumab might be next. Each drug works on a different pathway, so it’s worth trying another.
Do I need to stop other asthma medications when starting a biologic?
No. You keep using your inhaled corticosteroids and long-acting beta agonists. Biologics are add-on therapies. In fact, stopping your regular meds can make your asthma worse. Some patients reduce oral steroids over time, but only under medical supervision.
Michael Bene
December 3, 2025 AT 15:44Susan Haboustak
December 4, 2025 AT 00:10Cyndy Gregoria
December 4, 2025 AT 21:54Joanne Rencher
December 6, 2025 AT 07:01Cristy Magdalena
December 7, 2025 AT 18:26Wendy Chiridza
December 8, 2025 AT 01:18Pamela Mae Ibabao
December 8, 2025 AT 22:00Palanivelu Sivanathan
December 9, 2025 AT 16:57Gerald Nauschnegg
December 10, 2025 AT 03:59Erik van Hees
December 11, 2025 AT 08:58Chad Kennedy
December 13, 2025 AT 00:54Adrianna Alfano
December 14, 2025 AT 20:04Casey Lyn Keller
December 15, 2025 AT 19:27dylan dowsett
December 17, 2025 AT 14:04