Generic Absorption Rates: How They Must Match Brand Drugs to Ensure Safety and Effectiveness
When you pick up a prescription and see a different pill than last time - maybe a different color, shape, or even a different name on the label - it’s natural to wonder: Is this really the same? For millions of people taking generic medications, the answer is yes. But not because they look alike. Because they absorb the same.
What Does "Same Absorption" Really Mean?
A generic drug isn’t just a copy of the brand-name version. It has to prove it works the same way in your body. That means the active ingredient must enter your bloodstream at the same rate and in the same amount. This isn’t guesswork. It’s measured with strict science.The two key numbers doctors and regulators look at are AUC and Cmax. AUC stands for Area Under the Curve. It tells you how much of the drug your body absorbs over time - the total exposure. Cmax is the peak concentration, or how fast the drug reaches its highest level in your blood. If a generic drug’s AUC and Cmax values fall too far outside the brand’s, you might not get enough medicine, or you might get too much too fast.
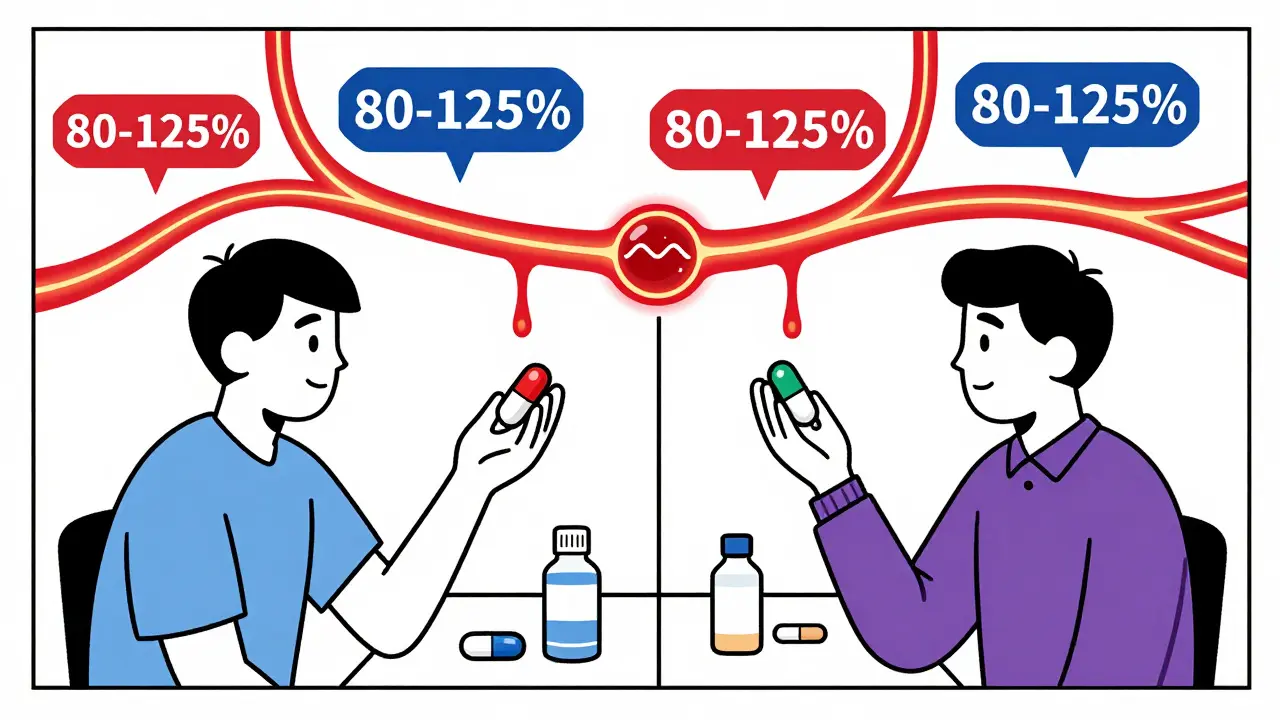
The FDA requires that the 90% confidence interval for both AUC and Cmax must fall entirely between 80% and 125% of the brand-name drug’s values. This isn’t a range of "allowed variation" - it’s a statistical guarantee that the average difference is tiny. In fact, a review of over 2,000 studies found that on average, generics differ from brand drugs by just 3.5% in absorption. That’s less than the natural variation you’d see if you took the same brand drug twice on different days.
Why 80-125%? It’s Not Arbitrary
You might think: Why not 90-110%? Why not 95-105%? The answer lies in biology.Even when you take the exact same brand-name pill, your body absorbs it differently each time. Factors like what you ate, how hydrated you are, your gut motility, or even your stress level can shift absorption by 10-15%. The FDA’s 80-125% range was chosen because it accounts for this natural variability. If a generic drug falls within this range, it’s not just "close enough" - it’s statistically indistinguishable from the brand in real-world use.
For most drugs, this works perfectly. But for drugs with a narrow therapeutic index - like warfarin, digoxin, or phenytoin - even a small shift can be dangerous. That’s why the FDA requires a tighter window: 90-111% for AUC. These drugs are in a special category. If you’re on one of them, your doctor may monitor your blood levels more closely, even if you’re on a generic.
What About Dissolution? Doesn’t That Matter?
You’ve probably heard that generics might dissolve slower or faster in your stomach. That’s true - and it’s also misleading.Studies show that more than half of generic drugs have different dissolution profiles than their brand-name counterparts. Some generics of nifedipine dissolve much slower. Others of amoxicillin dissolve faster. One study found that even different batches from the same manufacturer showed differences. So why aren’t these drugs pulled from shelves?
Because dissolution doesn’t always predict absorption. A pill might dissolve slowly in a test tube but still release its drug quickly in your gut. Or it might dissolve fast but get absorbed slowly due to how it interacts with your intestinal lining. The real test isn’t what happens in a lab - it’s what happens in your blood. And for nearly every generic approved by the FDA, the blood test - the in vivo study - proves it works.
The FDA doesn’t approve generics based on how they dissolve. They approve them based on what happens inside real human bodies. That’s why a generic that dissolves differently can still be perfectly safe and effective.
Do Patients Really Notice a Difference?
Yes - but not because the drug is different.On forums like Reddit and Inspire, people report that their generic bupropion "doesn’t work like the brand," or that their generic levothyroxine made them feel "off." A 2024 analysis of 1,247 patient reviews found that 12% reported perceived differences. Thyroid and antidepressant generics came up most often.
But here’s the catch: when researchers look at actual clinical data, they find no difference. A 2023 meta-analysis of 47 studies with nearly 10,000 patients showed no significant difference in outcomes between generic and brand-name cardiovascular drugs. The FDA has documented only 12 cases of possible therapeutic failure out of more than 14,000 approved generics since 2008. That’s a failure rate of 0.08%.
So why do people feel different? Sometimes it’s the pill itself - a different size, color, or even taste can trigger a psychological response. Sometimes it’s switching from one generic to another. A patient stabilized on a generic from Manufacturer A might switch to Manufacturer B, and even though both meet bioequivalence standards, their body may react slightly differently to the fillers or coatings. That’s not a failure of the system - it’s a reminder that human biology is complex.
What About the "B-Rated" Generics?
The FDA’s Orange Book assigns ratings to every approved drug. "A" means therapeutically equivalent. "B" means there’s a potential issue - maybe the drug hasn’t been studied enough, or there’s a formulation concern.Most generics are rated "A." But if a generic gets a "B" rating, it’s flagged. For example, some early generic versions of levothyroxine had inconsistent absorption between batches. Those were pulled. Today, the FDA requires stricter manufacturing controls for these drugs.
Experts recommend that if you’re already stable on a brand-name drug - especially for conditions like epilepsy, heart disease, or thyroid disorders - switching to a "B"-rated generic might not be the best move. But if you’re starting a new medication, a "B"-rated generic is still better than no treatment at all. The key is to work with your doctor and pharmacist to make sure you’re not switching back and forth between different generics unless necessary.

Why Do Generics Cost So Much Less?
Generics make up 90% of all prescriptions filled in the U.S., but only 23% of total drug spending. In 2023, the generic drug market was worth $135.7 billion. That’s because they don’t need to repeat expensive clinical trials. They just need to prove absorption matches.The cost savings are massive. The average copay for a generic is under $20. For brand-name drugs, it’s often over $50 - sometimes over $200. That’s why 90% of patients switch to generics when given the option. And when they do, they’re more likely to keep taking their medicine. A 2019 study found that patients abandon brand-name prescriptions at 266% higher rates than generics - not because they don’t trust them, but because they can’t afford them.
What’s Changing Now?
The FDA is moving toward smarter testing. Instead of always running human studies with 24-36 volunteers, they’re using computer models to predict how a drug will behave. This is called Modeling and Simulation (MIDD). It’s already being used for simple oral drugs. In the future, it might cut approval times from months to weeks.For complex drugs - like inhalers, topical creams, or injectables - the rules are still evolving. These don’t show up in blood tests the same way. So the FDA is developing new ways to measure their performance. But the goal hasn’t changed: make sure the medicine works the same way, no matter who makes it.
The bottom line? Generic drugs aren’t "almost as good." They’re proven to be just as good. The science is clear. The data is overwhelming. The system works - and it saves lives by making essential medicines affordable.
What Should You Do?
If you’re prescribed a generic:- Take it. It’s not a second-choice drug - it’s a scientifically validated alternative.
- If you notice a change in how you feel after switching, tell your doctor. Don’t assume it’s the drug. It might be a different filler, or a change in manufacturer.
- Don’t switch between multiple generics unless necessary. Stick with one that works.
- For narrow therapeutic index drugs, ask your pharmacist if your generic has an "A" rating in the Orange Book.
Generics aren’t a compromise. They’re a breakthrough. And the absorption rates? They’re not just close. They’re exact enough to keep you healthy - and save you money doing it.
Are generic drugs really as effective as brand-name drugs?
Yes, when approved by the FDA, generic drugs must prove they deliver the same amount of active ingredient into your bloodstream at the same rate as the brand-name version. Studies show the average difference in absorption is just 3.5%, which is less than the natural variation seen when taking the same brand drug twice. Over 90% of prescriptions filled in the U.S. are generics - and they’re used safely by millions every day.
Why do some people say generics don’t work as well?
Perceived differences are often due to psychological factors, changes in pill appearance, or switching between different generic manufacturers. While generics must meet strict absorption standards, they can differ in inactive ingredients like dyes or binders. These don’t affect how the drug works, but can cause minor reactions in sensitive individuals. Clinical studies consistently show no meaningful difference in outcomes between generics and brands.
What does the 80-125% range mean for generic absorption?
It’s the statistical window that ensures a generic drug’s absorption (measured by AUC and Cmax) is not meaningfully different from the brand. The 90% confidence interval for the ratio of the generic to brand drug must fall entirely within 80% to 125%. This accounts for normal biological variation in how people absorb drugs. Most generics fall within 95-105% - far tighter than the allowed range.
Can I trust a generic if it looks different from the brand?
Absolutely. U.S. trademark laws require generics to look different - different color, shape, or size - so they don’t copy the brand’s appearance. But appearance has nothing to do with how the drug works. The active ingredient, dosage, and absorption profile are identical. The FDA approves generics based on blood tests, not pill design.
Are there any drugs where generics might not be safe?
For drugs with a narrow therapeutic index - like warfarin, digoxin, phenytoin, and levothyroxine - even small changes in blood levels can be risky. The FDA requires tighter bioequivalence standards (90-111%) for these. Most generics for these drugs are still rated "A" and are safe. But if you’re stable on one brand or generic, avoid switching unless your doctor approves it. Always check the Orange Book for the FDA’s therapeutic equivalence rating.