Therapeutic Failures: When a Generic Drug Doesn't Work as Expected
It’s supposed to be the same drug. Same active ingredient. Same price. But for some patients, switching to a generic medication doesn’t just mean saving money-it means their condition gets worse, or they get sick in ways they never did before. This isn’t rare. It’s not an isolated case. It’s happening across the world, and the reasons go deeper than most people realize.
Why a Generic Might Not Work
Generic drugs are required to contain the same active ingredient as the brand-name version. That part is true. But here’s what most people don’t know: the FDA only requires generics to be bioequivalent-meaning the body absorbs between 80% and 125% of the active ingredient compared to the brand-name drug. That’s a 45% window. For most medications, that’s fine. But for drugs with a narrow therapeutic index (NTI), that margin is dangerous. NTI drugs include warfarin, phenytoin, levothyroxine, digoxin, and tacrolimus. These are medications where the difference between a therapeutic dose and a toxic one is razor-thin. A 10% drop in absorption might mean the drug doesn’t work. A 10% increase could cause poisoning. And under current rules, a generic could legally deliver 20% less or 25% more than the brand-right at the edge of what’s considered safe. In 2013, the FDA pulled Budeprion XL, a generic version of Wellbutrin, after hundreds of patients reported severe side effects: anxiety, dizziness, even seizures. The problem wasn’t the active ingredient. It was the inactive ones-the fillers, coatings, and binders that changed how the pill dissolved in the stomach. That small change turned a controlled-release tablet into a rapid-release one. The result? Too much drug too fast. Patients didn’t just feel worse-they got sick.The Silent Problem: Inconsistent Dosage
One of the most alarming findings came from a 2024 investigation by the Therapeutic Investigations Bureau of Journalism. They tested generic chemotherapy drugs used for breast, ovarian, and leukemia cancers. In some pills from the same batch, the active ingredient varied by more than 30%. One pill had 97% of the labeled dose. The next had 68%. That’s not a manufacturing error. That’s a systemic failure. Patients on these drugs weren’t just getting less treatment-they were getting unpredictable, dangerous doses. Some didn’t respond at all. Others developed severe organ damage. One patient, after switching to a generic version of methotrexate, ended up in the ER with vomiting so severe she couldn’t keep fluids down. Her cancer treatment had to stop. The tumor kept growing. Even more disturbing? This isn’t limited to overseas factories. In May 2024, Glenmark Pharmaceuticals recalled nearly 47 million doses of potassium chloride tablets because they weren’t dissolving properly. Patients with heart conditions who relied on these pills to regulate their electrolytes were at risk of sudden cardiac events. The problem? The tablets were too hard. They passed through the body intact.Who’s Responsible?
The generic drug supply chain is long, complex, and opaque. Most generics sold in the U.S. are made in India or China. Regulatory inspections are infrequent. In 2023, the FDA inspected only about 10% of overseas manufacturing facilities. Many facilities have multiple lines producing different drugs. One line might make a life-saving antibiotic. Another might make a generic blood pressure pill with inconsistent dissolution rates. Quality control isn’t always consistent across lines-or even across batches. A former FDA medical officer put it bluntly: “Valsartan is just the one we caught. Who knows how many more are out there?” That’s not fearmongering. It’s fact. Nitrosamine contaminants have been found in multiple blood pressure drugs-losartan, irbesartan, ranitidine. These aren’t accidental. They’re byproducts of poorly controlled chemical reactions. And they’re carcinogenic. Even when drugs pass regulatory tests, the real-world performance can differ. One study found that only 4 out of 12 generic versions of a popular ADHD medication dissolved at the same rate as the brand. Some dissolved over three times faster. That means the drug hits the bloodstream too quickly, causing side effects like jitteriness or insomnia. Others dissolve too slowly, leaving patients without adequate symptom control.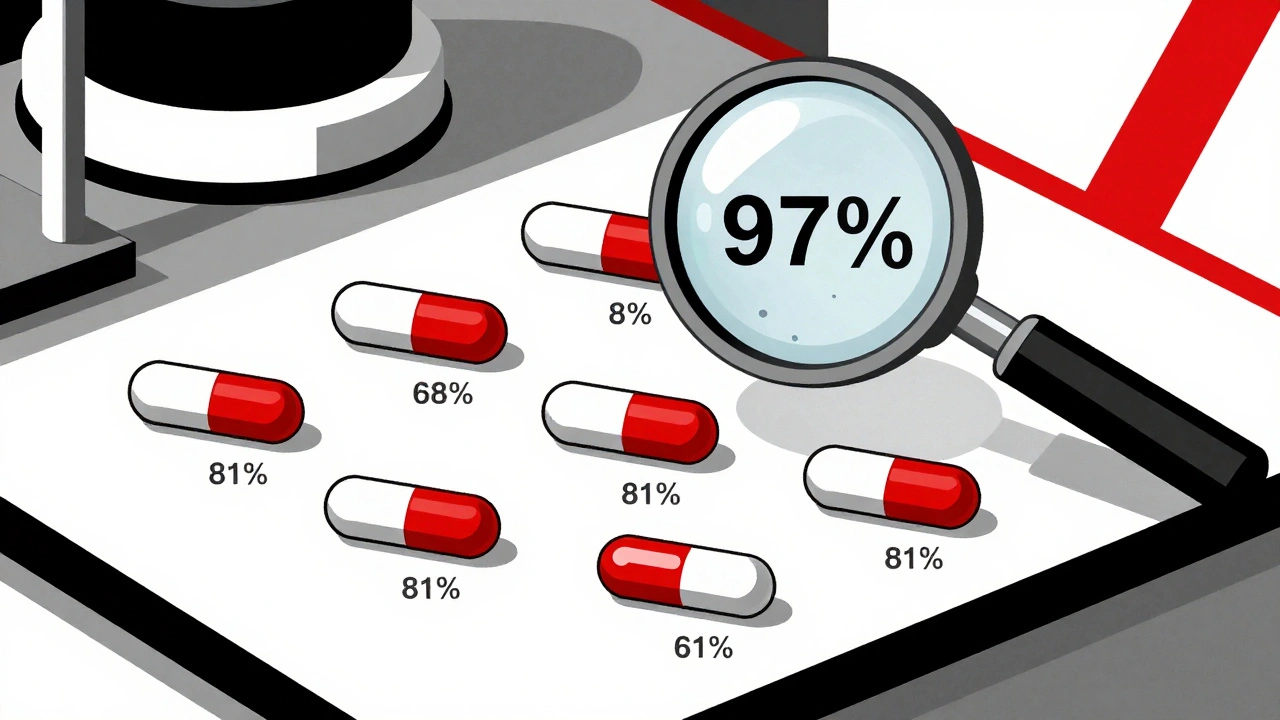
When It’s Not Your Fault
Patients aren’t to blame. Doctors aren’t always aware. Pharmacists are often pressured to substitute generics automatically. Insurance companies push for the cheapest option. But when a patient who’s been stable for years suddenly starts having seizures, or their thyroid levels go haywire, or their transplant starts rejecting-it’s not “just the disease progressing.” Doctors in the U.K. and U.S. have reported cases where switching from brand to generic triggered relapses in multiple sclerosis patients. One group of patients had generics containing 97% to 103% of the correct dose. Their disease stayed under control. Another group? Their generics had only 91%, 81%, and even 72% of the labeled dose. All of them relapsed within months. Salberg, a heart transplant patient, noticed her energy dropping after her pharmacist switched her tacrolimus to a generic. She felt fatigued, short of breath. Her doctors thought it was rejection. But when they switched her back to the brand, her symptoms disappeared. She later learned the generic had a different coating that slowed absorption. Her body wasn’t getting enough of the drug to prevent rejection.What You Can Do
If you’re on a medication with a narrow therapeutic index-warfarin, levothyroxine, phenytoin, cyclosporine, tacrolimus, or certain chemotherapy drugs-ask your doctor if the generic you’re taking has been tested for consistent performance. Don’t assume it’s the same. Keep a log: note when you switch brands, when symptoms change, when side effects appear. Bring this to your doctor. If you notice a pattern, request the brand-name version. Many insurers will approve it if you can show a documented therapeutic failure. Pharmacists can’t always tell you which manufacturer made your generic. But you can ask. Look at the pill imprint or the pharmacy label. If you’ve had a good experience with one brand of generic, ask to stick with it. Consistency matters. If you’re on a drug that’s been recalled-like ranitidine, valsartan, or potassium chloride-check the FDA’s recall list. Don’t rely on your pharmacy to notify you. They’re often behind.
Annie Grajewski
December 5, 2025 AT 06:41so like… i took this generic levothyroxine and started feeling like a zombie who forgot how to breathe? turns out the batch i got had 78% of the labeled dose. my endo was like ‘maybe its stress’ lol. no. its the pill that’s broken. also why does my pill look like a tiny beige brick? the brand one was smooth as silk. also why is no one talking about this??
Mark Ziegenbein
December 6, 2025 AT 03:39Let me be perfectly clear-this isn't about generics. It's about the collapse of regulatory integrity in a neoliberal healthcare system that treats human life like a spreadsheet line item. The FDA, underfunded and politically neutered, rubber-stamps foreign-manufactured pills while CEOs pocket billions. The 80-125% bioequivalence window isn't a scientific standard-it's a corporate loophole dressed in bureaucratic legalese. And don't get me started on how pharmacies are legally mandated to substitute without patient consent. This is pharmaceutical eugenics disguised as cost-saving. We're not talking about aspirin here. We're talking about drugs where a 10% variance can kill you. And yet the system shrugs. Because the people who die? They're not shareholders.
Norene Fulwiler
December 8, 2025 AT 02:22I’m a nurse in a rural clinic and I’ve seen this over and over. One woman switched from brand to generic tacrolimus after her insurance denied coverage. Within weeks, her transplant numbers went off the charts. She didn’t know what was happening. No one explained the risks. She thought it was just ‘bad luck.’ We had to scramble to get her back on the brand. She cried when she found out it wasn’t her body failing-it was the system. We need better communication. We need transparency. And we need to stop treating patients like numbers.
William Chin
December 8, 2025 AT 05:21It is imperative that regulatory agencies enforce stringent, non-negotiable bioequivalence thresholds for all narrow-therapeutic-index pharmaceuticals. The current standards represent a gross dereliction of duty. Furthermore, the lack of mandatory batch-level traceability constitutes a violation of patient autonomy and informed consent. It is not merely an oversight-it is an ethical catastrophe. I urge all stakeholders to petition Congress for immediate legislative reform under the auspices of the Food, Drug, and Cosmetic Act. Patient safety cannot be commoditized.
James Moore
December 8, 2025 AT 16:58Look, I get it-America’s got the best healthcare in the world… unless you’re poor. Then you get pills made in some factory where the quality control guy is napping while the machine spits out tablets that dissolve in 3 seconds or never dissolve at all. Meanwhile, China’s got better drug safety standards than we do. And India? They’re making billions off our broken system. We let corporations cut corners so we can save $5 on a pill… and then someone ends up in the ER with a heart attack because their potassium tablet didn’t dissolve. This isn’t capitalism-it’s a death sentence with a discount sticker.
Kylee Gregory
December 10, 2025 AT 14:20I think what’s really missing here is the human layer. Behind every pill is someone who’s been stable for years-maybe they’re a parent, a teacher, a veteran-and then one day, they’re not. The system doesn’t see them. It sees cost savings. But what if we treated these drugs like we treat insulin? Like we treat heart meds? Like we treat anything that keeps someone alive? Maybe then we’d stop treating bioequivalence like a math problem and start treating it like a promise.
Laura Saye
December 11, 2025 AT 05:19The pharmacokinetic variability in NTI generics introduces a profound epistemic uncertainty for patients-where the therapeutic effect becomes stochastic rather than deterministic. This creates a latent state of iatrogenic anxiety, where the body becomes an unpredictable variable. The psychological toll is often underappreciated: the constant monitoring, the fear of dosing inconsistency, the erosion of trust in medical authority. It’s not just a physical crisis-it’s a crisis of embodied safety.
luke newton
December 12, 2025 AT 15:01People like you are the reason this country is falling apart. You whine about generics while your lazy, entitled self won’t pay $10 more for a brand-name drug. If you can’t afford it, maybe you shouldn’t be on expensive meds. Stop blaming the system. Take responsibility. And if you’re too weak to handle a little variability, maybe you’re not cut out for real life.
Ali Bradshaw
December 12, 2025 AT 21:28My mate in the UK had the same issue with his generic phenytoin-seizures came back after the switch. He got his GP to fight the system, and they finally approved the brand after he showed his blood level logs. Point is: it’s possible to push back. Keep records. Ask for the batch number. Don’t take ‘it’s the same’ as an answer. You’re not being difficult-you’re being smart. And if your doc doesn’t get it, find one who does. You’ve got this.