Tag: bioequivalence
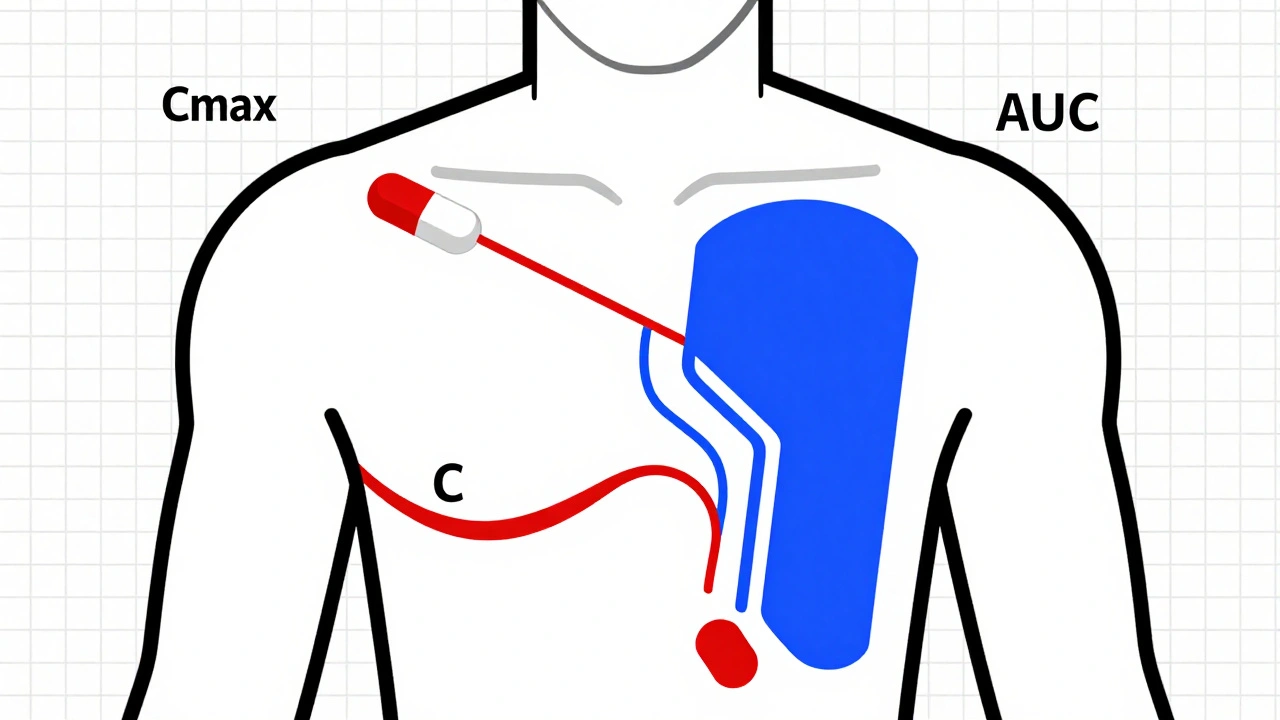
Pharmacokinetic studies are the main way regulators prove generic drugs work like brand-name versions. But they’re not perfect. Learn how they work, where they fail, and what’s replacing them for complex drugs.
- Read More